Developmental Pathophysiology of Somatic Mutations in Neocortex
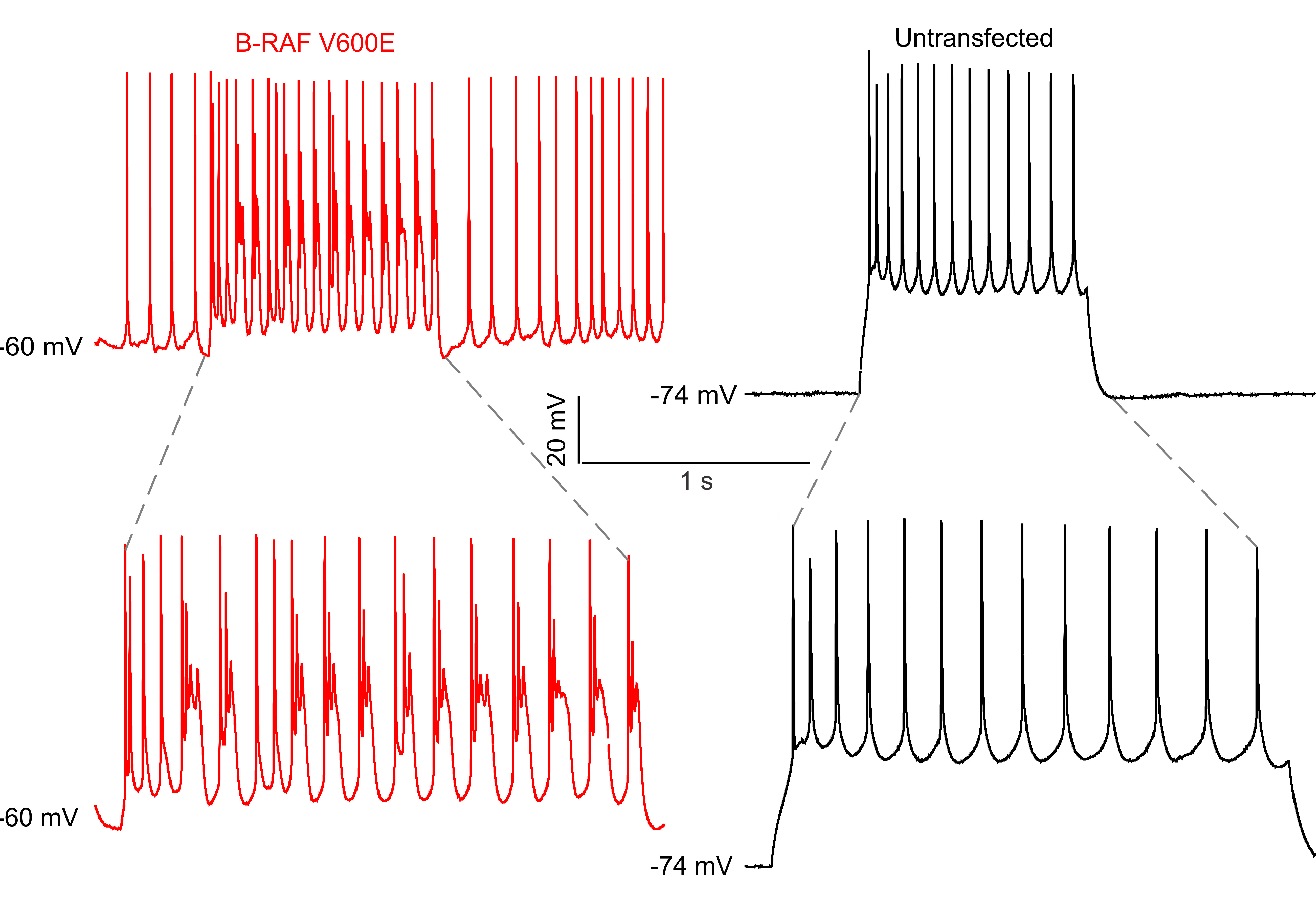
Every cell has a potentially unique combination of mutations accumulated by imperfect DNA repair. If somatic mutations occur early in cerebral cortical development they can impact function. The best demonstrated example of somatic mutations causing pathophysiology is seizures caused by glial-neuronal tumors and focal cortical dysplasias. The most common somatic mutations identified to date include activating BRAF kinase mutations, and activating mutations in MTOR, AKT, and PIK3CA kinases in focal cortical dysplasias. What remains largely unknown is precisely how and whether different somatic mutations lead to neuronal hyperexcitability in cortical neurons and hypersynchrony in cortical circuits. Using novel animal models of focal somatic mutation in neural progenitors we are testing three hypotheses focused on defining the underlying developmental, cellular and molecular causes of seizures resulting from somatic mutations in cortex: 1) cellular phenotypes and seizure severity are a function of the sub-type of neocortical progenitor in which epileptogenic mutations arise, 2) elevated cortical excitability is a direct consequence of overactive MAPK/ERK and MTOR pathways in either or both neurons and astrocytes, and 3) epileptiform activity spreads from perilesional zones by altered connections to inhibitory interneuron networks that result in hypersynchronous activity in and seizure propagation from perilesional zones.
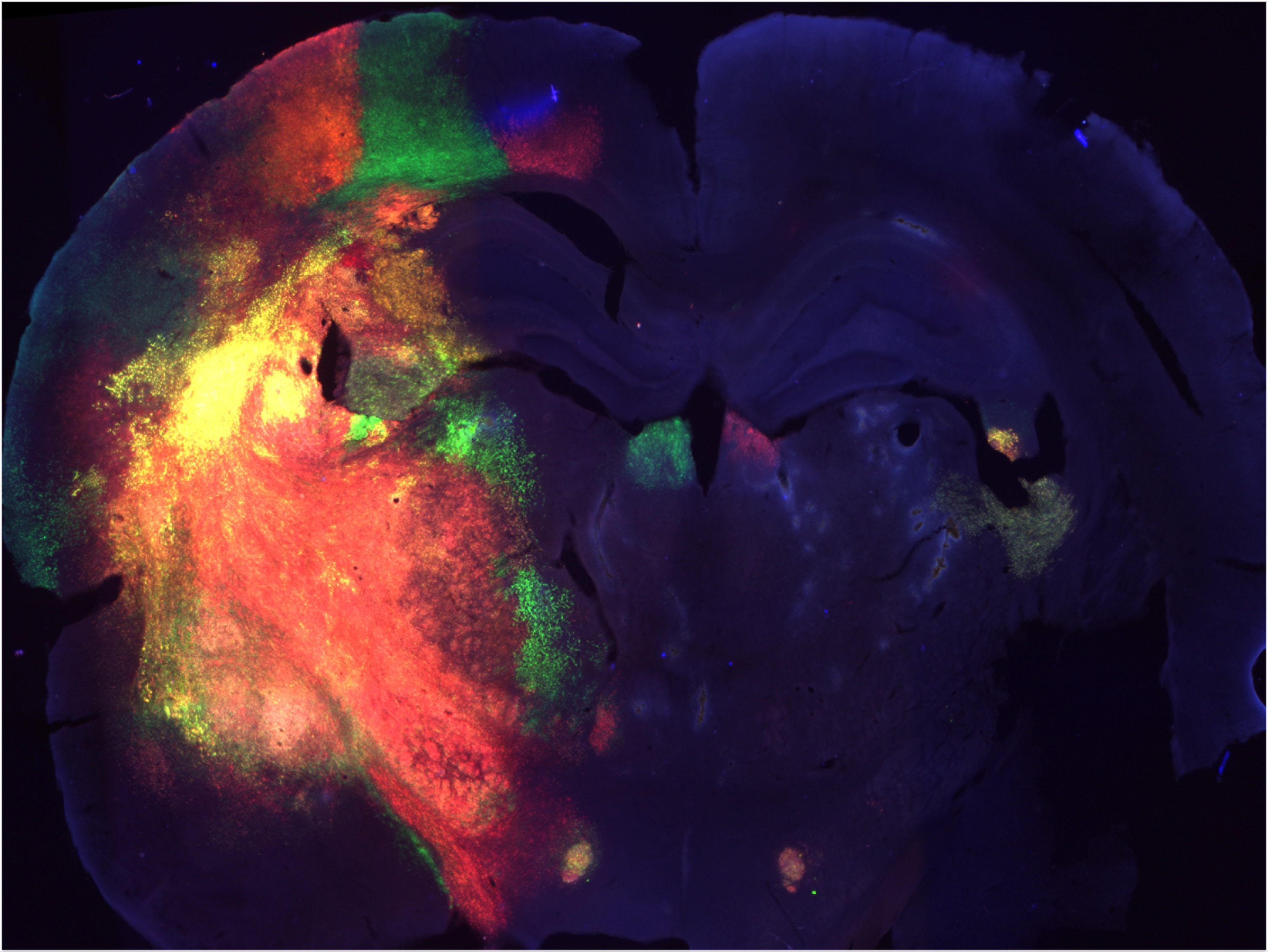
CRISPR Models of Pediatric Glioma
Tools for inducing targeted somatic cell mutation are important for modeling and studying human disease and disease processes including cancer. The CRISPR/Cas9 system is a powerful tool for targeted genome engineering, and its application in developing nervous system cells in vivo has become a powerful approach for generating novel model of tumors in developing brain. We have tested the application of the CRISPR/Cas9 system to target three tumor suppressor genes (PTEN, NF1, and P53) alone and in combination. We showed that gRNAs targeting PTEN successfully abolish PTEN expression in neurons and leads to neuronal phenotypes consistent with PTEN loss of function including neuronal hypertrophy and altered neurophysiology. We further showed that targeting NF1 leads to pronounced glial proliferation, and if three tumor suppressors are simultaneously targeted by multiplex CRISPR/CAS9, then glioblastoma multiforme tumors form. Our results demonstrate that the CRISPR/Cas9 system can be used to efficiently target combination of genes in developing brain to generate novel models of pediatric glioma.